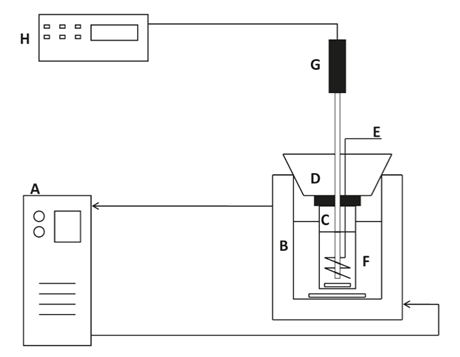
The freezing points are measured using a modified Beckmann apparatus. The experimental setup is shown in the figure.
The temperature is controlled by a Lauda RE 110 thermostatic bath, which is able to lower the temperature of the refrigerant to approximately 233 K (A). Ethanol is used as refrigerant. The sample temperature is recorded by an Agilent 34970A data acquisition unit in connection to a PC (H) using a Pt100 DIN 1/10 custom-made Beta temperature sensor (G).
It is made with a handle, 30 cm in length, and a 0.8 cm Pt100 element pushed inside a stainless steel container to the tip of the probe. The small element insures that the temperature is measured in the liquid sufficiently far away from the surface.
Freezing point measurements are carried out in a sample glass (C) fitted with a magnetic stirrer, a device for manual stirring (E), as well as the temperature sensor (G). The lid of the container is mounted to a large rubber stopper (D). Both lid and the rubber stopper are penetrated by the temperature probe and magnetic stirrer.
The total pressure is the atmospheric pressure under the experimental conditions. The sampling glass container is placed in a controlled temperature bath (F). The constant temperature is maintained by the cooling jacket (B).
An additional magnetic stirrer is placed in the cooling liquid in order to ensure homogeneous temperature conditions outside the sample glass. The Agilent data acquisition unit is calibrated against recommended freezing point values of aqueous NaCl.
Seven sample solutions for the calibration are prepared in the concentration range from 0 to 0.2 mass fraction with freezing points between (0 and 253.15) K (-20 °C). 10-fold measurements are made of the calibration samples. The standard deviation of the calibration measurements does not exceed 0.03 K.
Publications
Philip Loldrup Fosbøl, Randi Neerup, Muhammad Waseem Arshad, Zacarias Tecle, and Kaj Thomsen, "Aqueous Solubility of Piperazine and 2-Amino-2-methyl-1-propanol plus Their Mixtures Using an Improved Freezing-Point Depression Method,"
J. Chem. Eng. Data, 2011, 56 (12), p. 5088–5093, 2011
Philip Loldrup Fosbøl, Mikkel Gielsager Pedersen, and Kaj Thomsen, "Freezing Point Depressions of Aqueous MEA, MDEA, and MEA-MDEA Measured with a New Apparatus", J. Chem. Eng. Data 56, p. 995-1000, 2011