B.Eng. Project entitled Hydrophilic materials for the generation of the fourth phase of water (in collaboration with a US company)
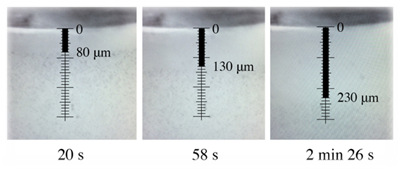
A result from this project: The figure below shows the formation of an exclusion zone in the proximity of a round Nafion slice.
The round Nafion slice is seen at the top of the figure. The clean zone is seen to grow over time, and the “impurities”, consisting of polystyrene microspheres, are seen to have moved away from the Nafion surface, whereas a zone with clean water is created.
The EZ zone developed immediately and reached a maximum distance of app. a quarter of millimeter, as seen below.
Student special course on Structured Water in relation to solid surfaces
Water-Bridge Experiment
Water structure and hydrogen bonding
[For more information, see Liang, X.D., Maribo-Mogensen, B., Tsivintzelis, I., Kontogeorgis, G.M., 2016. A comment on water’s structure using monomer fraction data and theories. Fluid Phase Equilibria, 407: 2-6]
Advanced thermodynamic models, which are very popular in the 21st century, are often expressed in the form of equations of state, and they describe quantitatively the hydrogen bonding (HB) effects.
In the usual terminology, this means we can calculate the monomer fraction (percentage of molecules not present in HBs) as a function of e.g. temperature or composition (for mixtures), as can be seen in the figure below (modified from Liang et al., 2016).
These predictions can be compared against spectroscopic data, e.g. those presented by Luck [1] for water. It can be seen that all models and the recent molecular simulation data predict much more HB (less fraction of monomers) than the Luck spectroscopic data.
Models and molecular simulation agree well with each other, but not with Luck data. What makes the situation even more blurry is that more recent spectroscopic (IR, NMR) and X-ray and neutron diffraction data [5] for water HB predict even higher monomer fraction than Luck’s data, thus in complete contradiction to otherwise successful thermodynamic theories.
If, on the other hand, thermodynamic theories are forced to represent the Luck monomer fraction data, other properties such as phase behavior are poorly reproduced. Another serious problem is that with this uncertainty in the measurements, it is difficult to obtain a true understanding of the exact nature of hydrogen bonding of water and how it interacts with other molecules.
Much more research is needed to understand the situation !
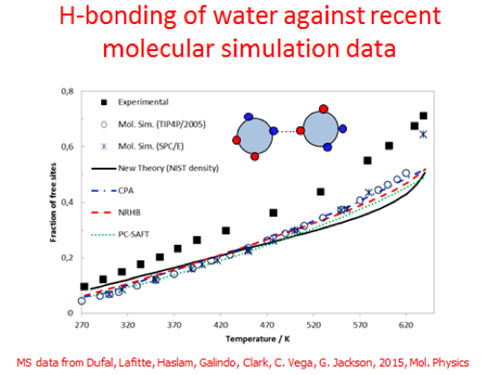
Free site fraction of saturated water using thermodynamic models (CPA, PC-SAFT, NRHB), experimental data, the new theory (Maribo-Mogensen et al., [2,3]) and recent molecular simulation data from Dufal et al. [4]. The experimental (NIST) density is used for water as input in the new theory. The experimental spectroscopic data are from Luck [1].
1. Luck, W. A. P. A, 1980. Model of Hydrogen-Bonded Liquids. Angew. Chem. Int. Ed. Engl., 19: 28.
2. Maribo-Mogensen, B., Kontogeorgis, G. M.; Thomsen, K., 2013. Modeling of dielectric properties of complex fluids with an equation of state. J. Phys. Chem. B., 117(12): 3389-3397.
3. Maribo-Mogensen, B., Kontogeorgis, G. M.; Thomsen, K., 2013. Modeling of dielectric properties of aqueous salt solutions with an equation of state. J. Phys. Chem. B., 117(36): 10523-33.
4. Dufal, S., Lafitte, Th., Haslam, A.J., Galindo, A., Clark, G.N.I., Vega, C., Jackson, G., 2015. The A in SAFT: developing the contribution of association to the Helmholtz free energy within a Wertheim TPT1 treatment of generic Mie fluids. Molecular Physics, 113(9-10): 948-984.
5. Mallamace, F., Branca, C., Broccio, M., Corsaro, C.,Mou, C.-Y., Chen, S.-H., 2007. The anomalous behavior of the density of water in the range 30 K < T < 373 K. PNAS, 104 (47): 18387