Systems containing associating molecules are non-ideal and difficult to model. Quantum chemistry is applied to quantify intermolecular interactions and improve thermodynamic models for these challenging systems.
Thermodynamic models that explicitly account for association have become essential tools for correlating and predicting multiphase equilibria.
These models have many adjustable parameters which makes it difficult to uniquely correlate experimental data.
Reducing the number of adjustable parameters is an important pathway to increasing the reliability and extrapolation power of thermodynamic models for practical applications.
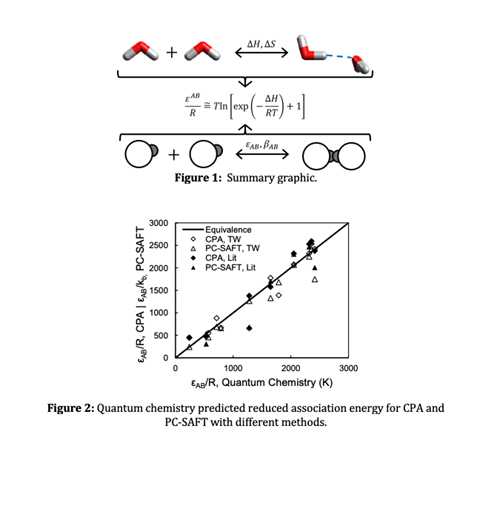
The chemical and perturbation theories of association are related which creates a pathway for estimating association parameters using quantum chemistry calculations.
Parameters estimated from these relationships are applied to model pure components and mixtures with the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) and Cubic Plus Association (CPA) equations of state.
Main supervisor:
Georgios Kontogeorgis
Co- supervisor:
Xiaodong Liang